Q LEAF PRO
Coronary valves made from animal heart tissue much more efficient and durable
According to the American Heart Association, the global cost of treating severe coronary heart disease is expected to double by 2030, reaching a value of USD 70 billion. To meet these challenges, cutting-edge companies are designing more efficient and less expensive medical devices.
In this context, LVD Biotech, Sensofar Medical, and the UPC-CD6 collaborate in the Q LEAF PRO project, with the aim of optimising the manufacturing process and inspection of coronary valves made from animal tissue, thus improving their efficiency and durability.
The initiative, coordinated by secpho, has received funding from the Ministry of Industry, Trade and Tourism corresponding to Next Generation EU funds, canalised through the support programme for Innovative Business Groups, whose objective is to digitalise industry.
More than 10 million stents and 100,000 coronary valves were successfully implanted worldwide in 2018, and the forecast for this market is for further growth.
Medical technology is evolving rapidly. Healthcare systems face critical challenges in developing better and less costly therapies that have a positive impact on people’s health and lives.
According to the American Heart Association, the global cost of treating severe coronary heart disease is expected to double by 2030, reaching a value of USD 70.000 million. To meet these challenges, cutting-edge companies are designing more efficient and less costly medical devices. In this context, implantable vascular devices, such as stents and, more recently, coronary valves, are having a huge impact. In 2018, more than 10 million stents and 100,000 coronary valves were successfully implanted worldwide, and the forecast for this market is to continue to grow.
More than 30% of patients with severe aortic stenosis are not suitable for surgery, as it carries a very high risk.
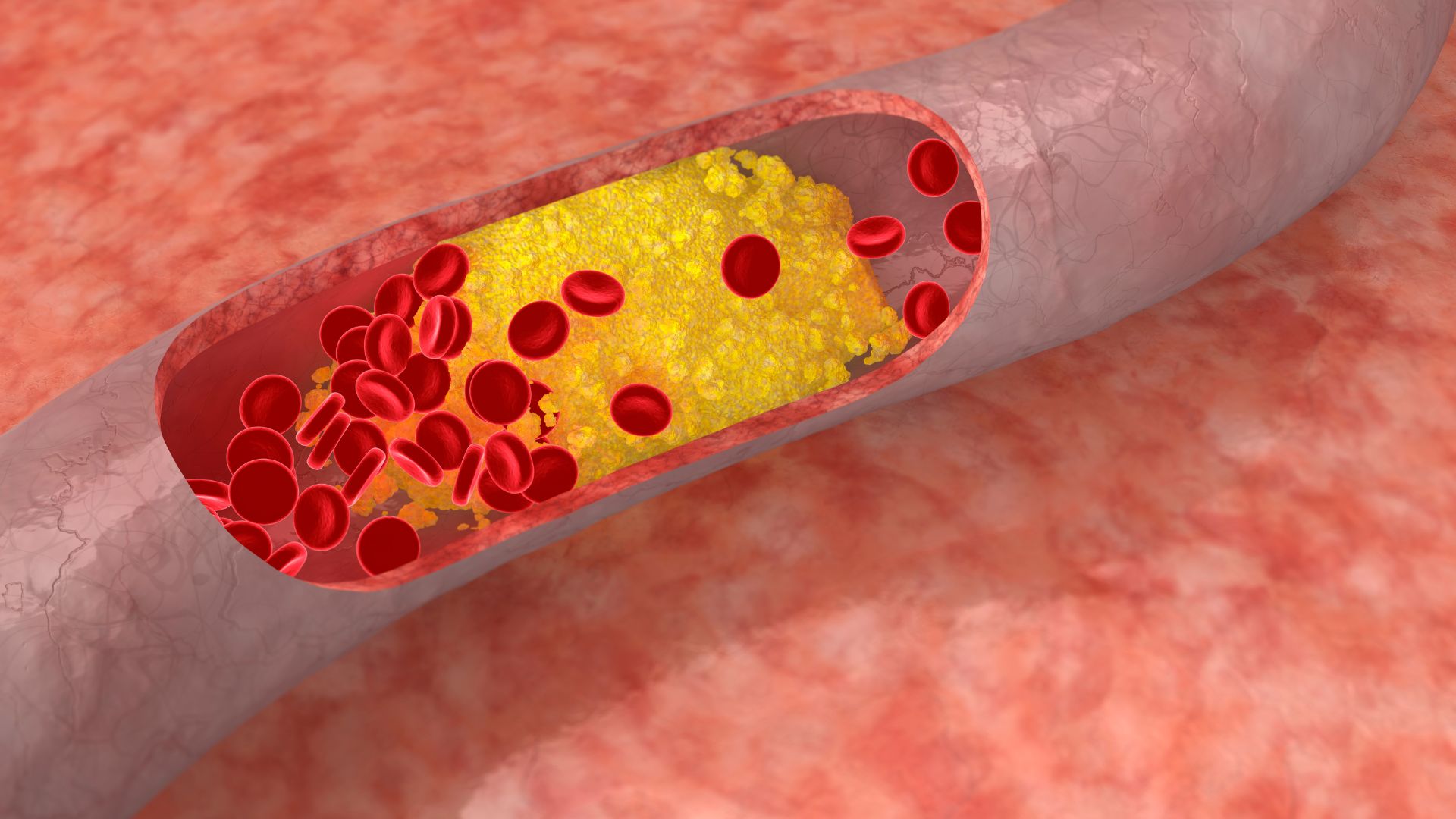
An increasingly important and very common coronary artery disease (> 20%) in elderly patients is severe aortic stenosis. This occurs when the aortic valve of the heart narrows, blocking blood flow from the heart to the main artery of the body (aorta) and to the rest of the body. This blockage can cause serious problems such as heart attacks, strokes and even death. With a mortality rate of 25% at one year and 50% within two years of the onset of symptoms, most patients are asymptomatic and have a very rapid progression.
Valve replacement surgery has proven for many years to be a satisfactory short- and long-term solution even in very elderly patients. However, more than 30% of patients with this pathology have never been considered suitable for surgical treatment, in most cases because their cardiologist considers the risk to be unacceptably high for conventional surgery.
A very recent alternative to conventional valve replacement surgery is provided by the new sutureless coronary valves (HV) and transcatheter valves (TAVIs). Sutureless valves allow high-risk patients to be operated safely and with the best results. The process is faster, safer and less surgically invasive.
A very recent alternative to conventional valve replacement surgery is provided by the new sutureless coronary valves (HV) and transcatheter valves (TAVIs). Sutureless valves allow high-risk patients to be operated safely and with the best results. The process is faster, safer and less surgically invasive.
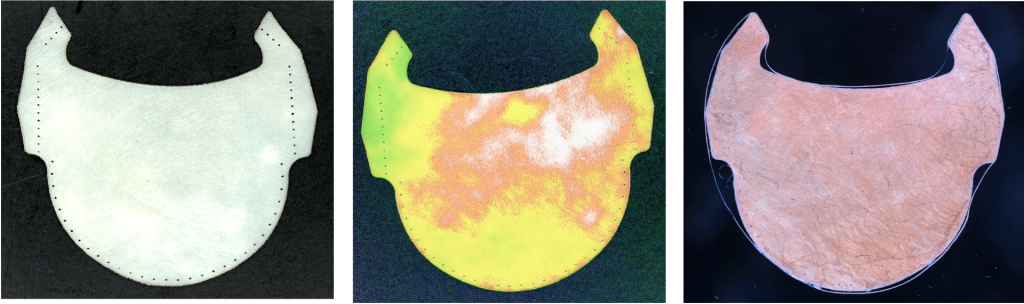
QLEAF PRO digitises, selects and validates animal pericardial tissues to make more efficient and durable heart valves.
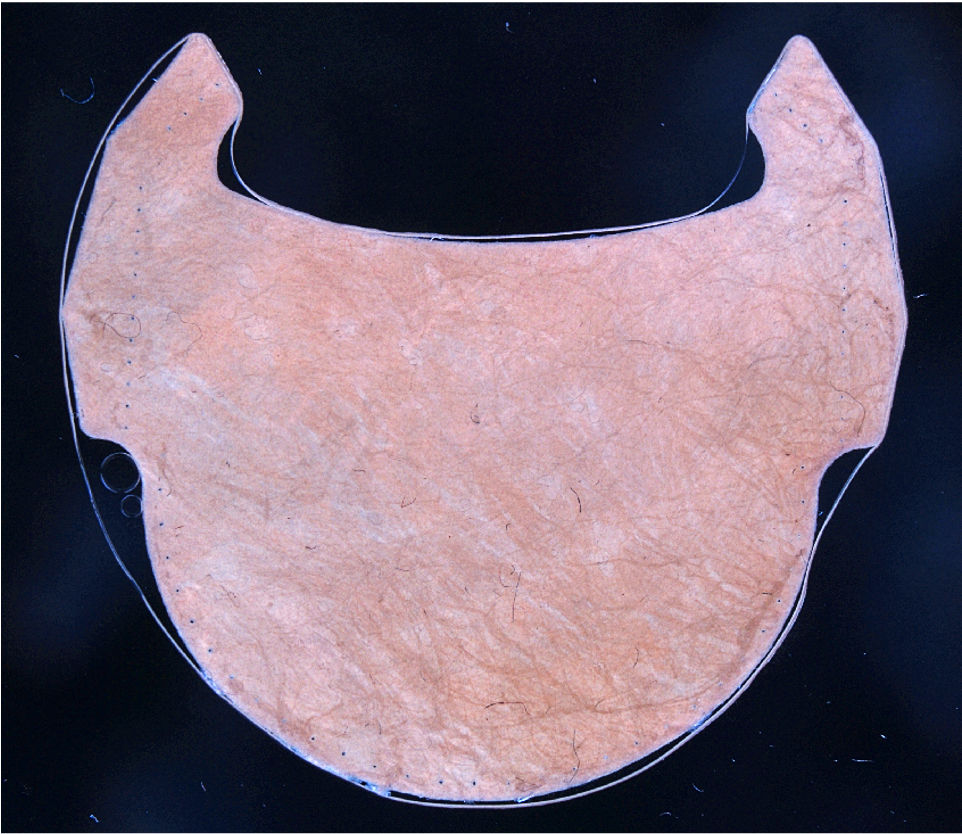
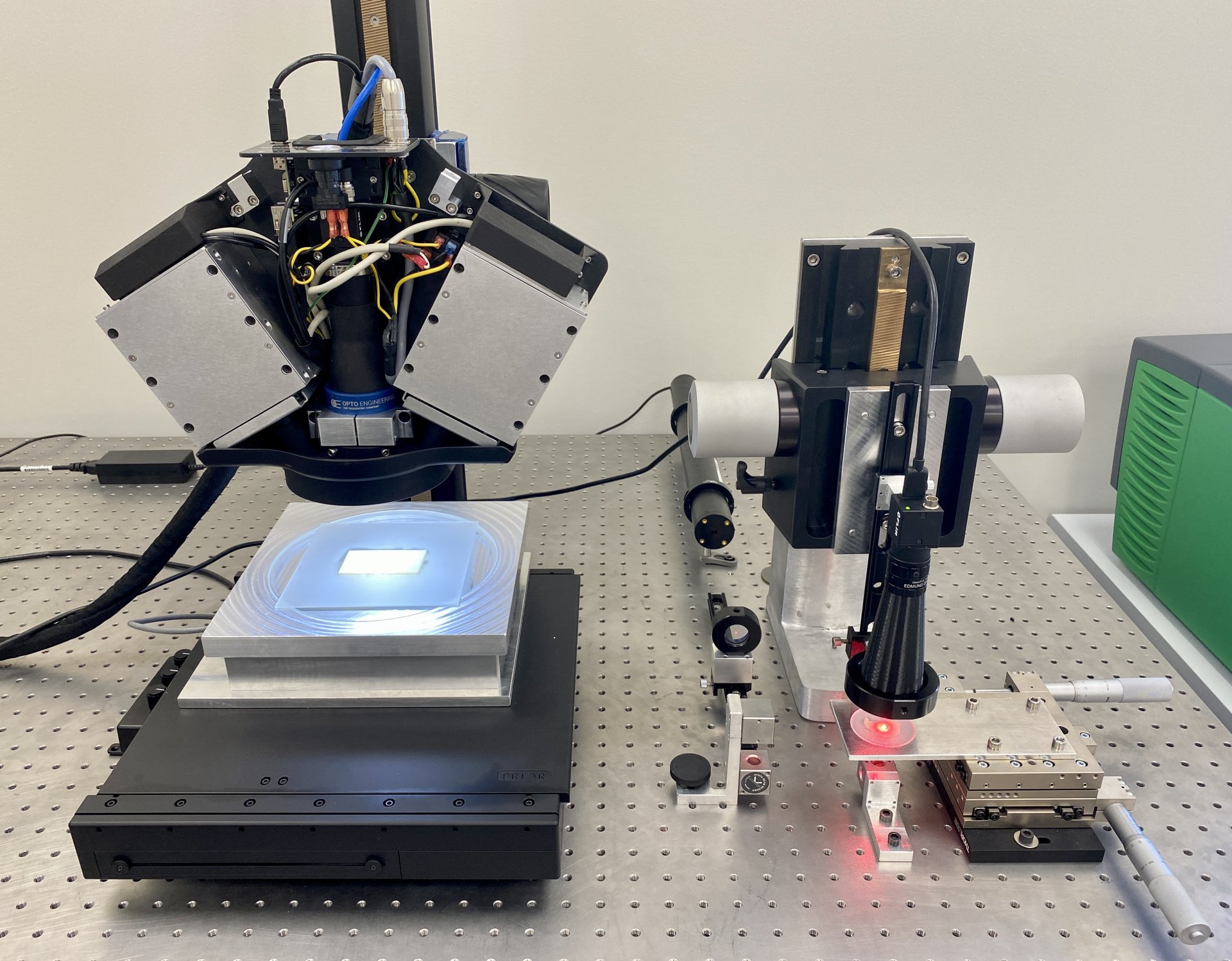
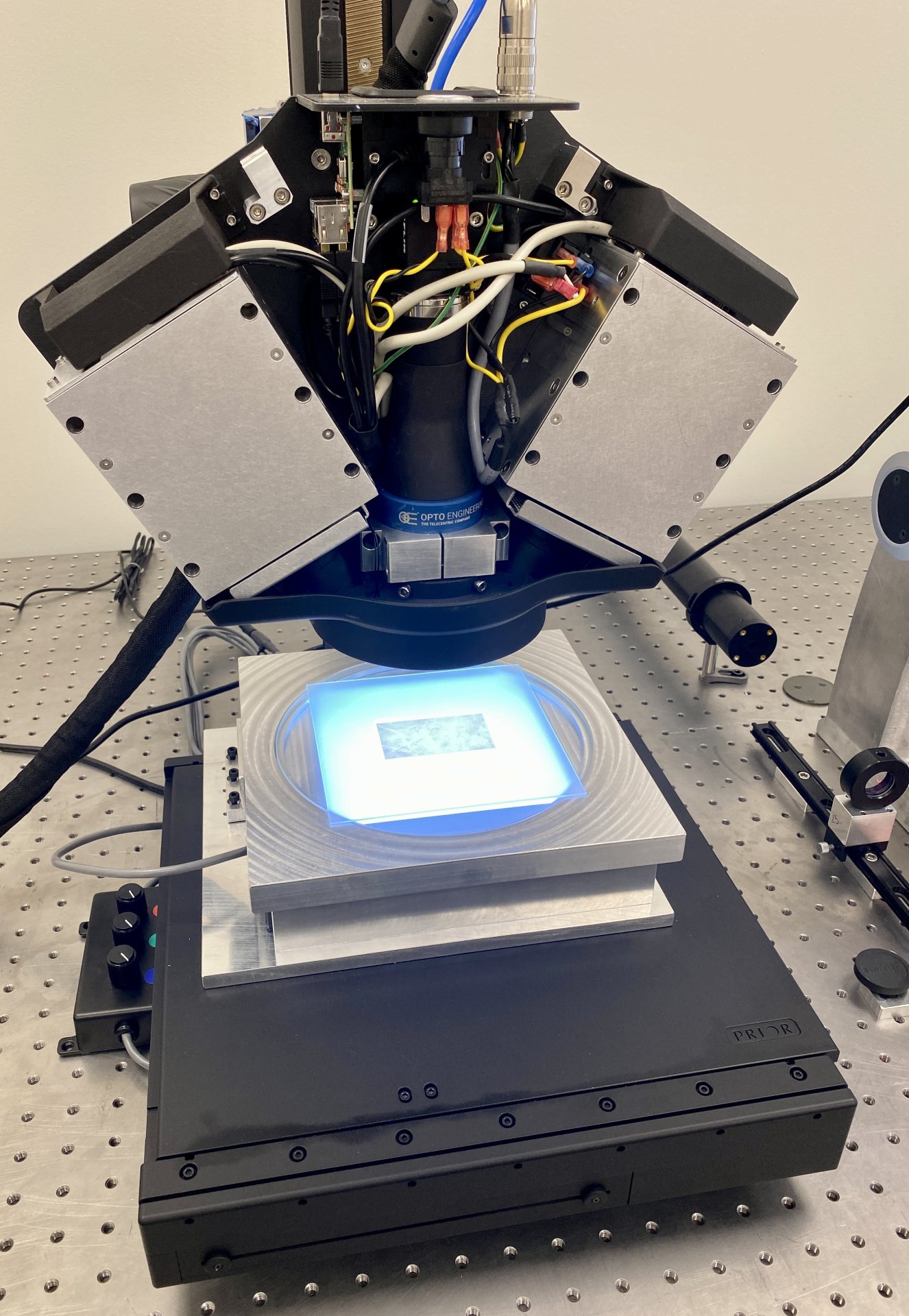
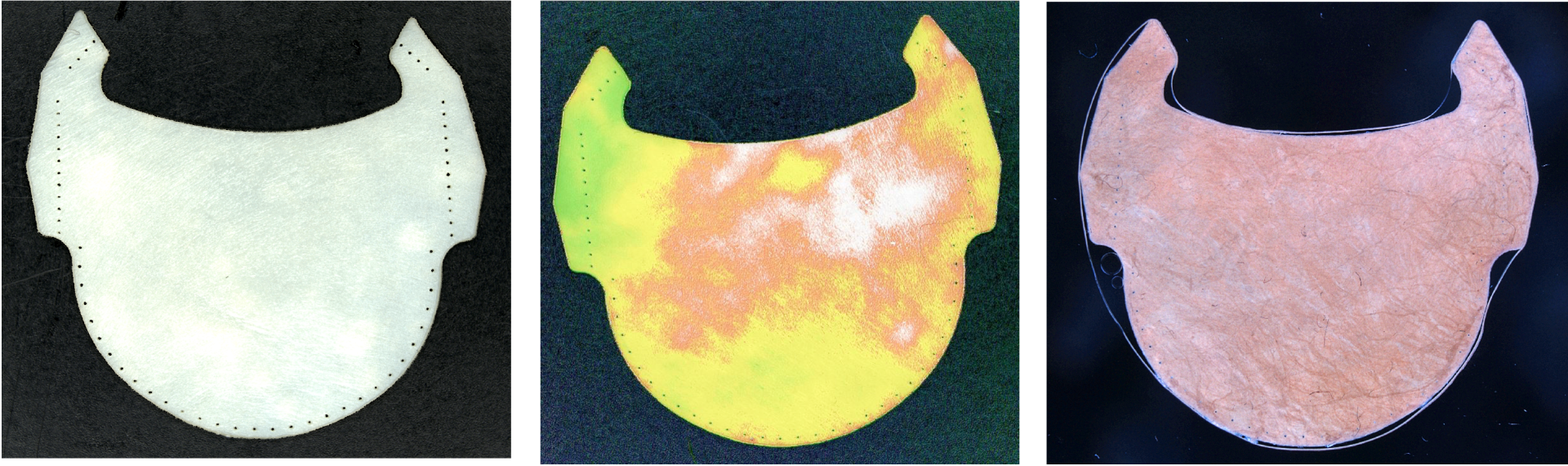
In this context, the Q LEAF PRO project aims to optimise the manufacturing and inspection processes of the organic part of the valve, i.e. the leaflets, in order to manufacture much more efficient and durable coronary valves. Specifically, a flexible and intelligent cell based on optical sensors is being developed to digitise animal pericardial tissues, automatically select the optimal regions to be used as membranes or leaflets in bioprosthetic coronary valves and proceed to their automatic manufacture and final quality control.
With the execution of this project, it has been possible to develop a physical prototype of the manufacturing cell, from which a potential end user can evaluate the quality of the leaflets obtained automatically and, consequently, validate the technologies used in the critical stages of the manufacturing process. In addition, the prototype has also made it possible to estimate the productivity of the cell and the manufacturing costs of the leaflets.